Regulatory Services for Medical Devices
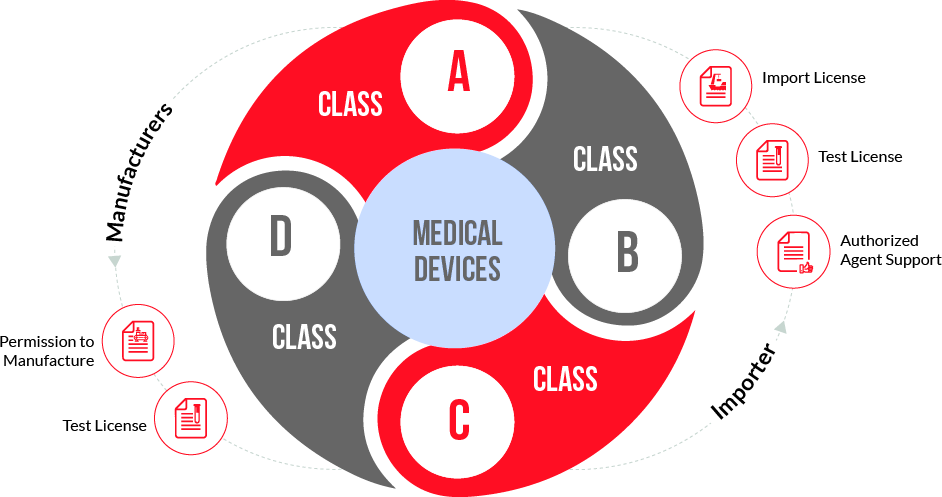
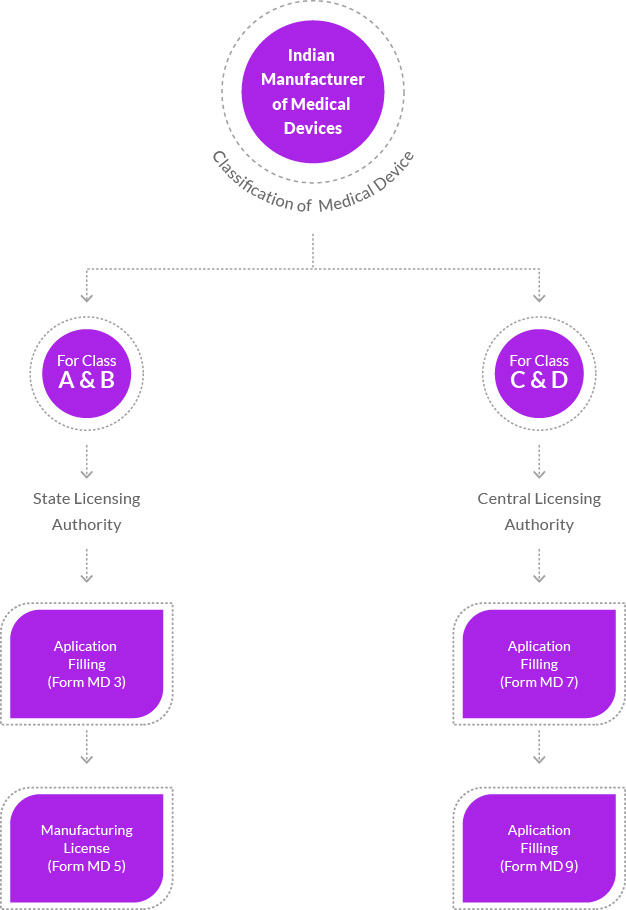
The Indian government has consistently regulated and monitored medical devices due to their critical nature. With the continuous evolution of medical technology and the escalating demand for such devices in the country, new regulations and reforms have been implemented. These devices are now classified into categories A, B, C, and D, each overseen by relevant central and state regulatory authorities. Classification is based on the associated risk, and streamlined procedures for license applications have been established. The government has also instituted rules and guidelines for the import of medical devices, requiring the involvement of an authorized Indian agent for license applications.
Thanks to the government's proactive measures, like the SUGAM portal, acquiring licenses for medical devices has become a more efficient process. At ZYPS, we are dedicated to offering regulatory services for medical devices, providing support to our clients in navigating through application and licensing procedures.