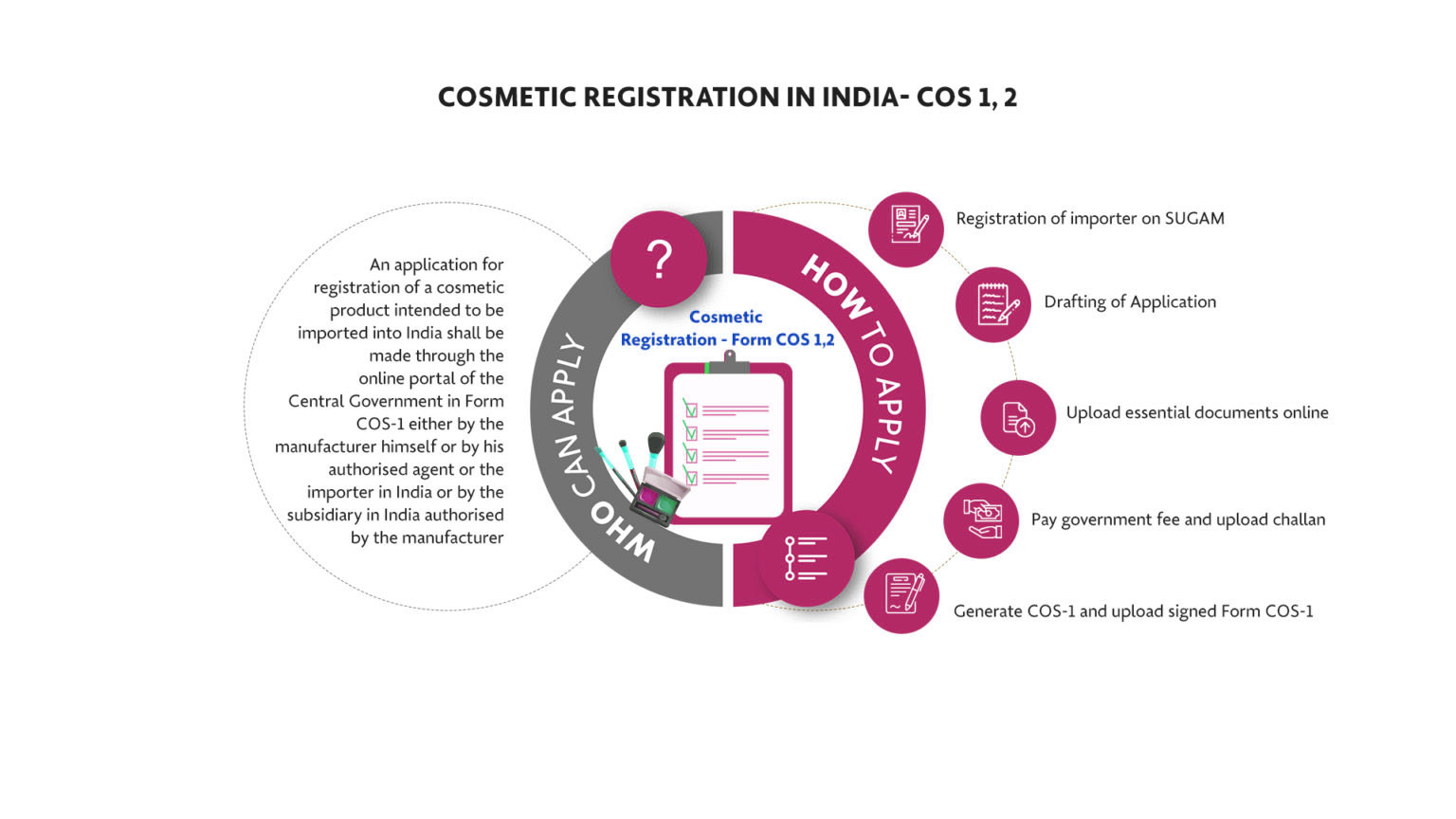
COSMETIC REGISTRATION
Certificate in India : COS-1 & COS- 2
Secure a refreshed cosmetic registration certificate using the Cos 2 form with professional assistance from ZYPS. We offer expert guidance on strategic product planning and testing to obtain the import license for cosmetics (Cos 2/Form 43) in India through CDSCO.