CDSCO
Manufacturing Services for In-Vitro Diagnostics
ZYPS has assisted over 30 In-Vitro Diagnostics (IVD) manufacturers to date, seamlessly managing all regulatory and compliance requirements.
Contact UsZYPS has assisted over 30 In-Vitro Diagnostics (IVD) manufacturers to date, seamlessly managing all regulatory and compliance requirements.
Contact UsDiscover a holistic approach to consulting services designed specifically for the In-Vitro Diagnostics (IVD) sector. Our team brings a wealth of experience and expertise to every project, ensuring that your unique needs are met with precision and care. From regulatory compliance to market strategy, we offer a full spectrum of services to support your IVD endeavors. With a proven track record of success and a commitment to excellence, we stand ready to guide you through every step of the process. Partner with us and gain a competitive edge in the dynamic landscape of IVD innovation.
The Central Drugs Standard Control Organization (CDSCO) oversees the regulation of manufacturing, importation, and distribution of In-Vitro Diagnostic (IVD) devices within India. It has established a comprehensive regulatory framework that mandates adherence to various requirements encompassing registration, classification, clinical performance evaluation, labeling, adverse event reporting, and post-market surveillance for IVD manufacturers. Compliance with these regulations is crucial to ensure the safety and efficacy of IVD devices available in the Indian market.
CDSCO's pivotal role involves enforcement of regulations, continuous monitoring of IVD device quality and safety standards, and taking necessary actions in instances of non-compliance. By upholding stringent regulatory standards, CDSCO safeguards the well-being of the Indian populace, promoting confidence in the safety and effectiveness of IVD devices circulating in the country.
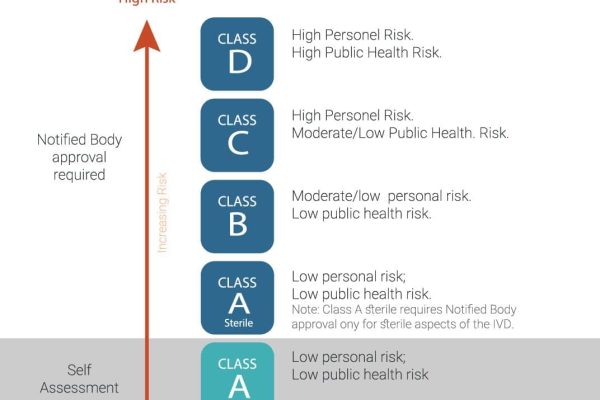
TheIn accordance with the New Medical Rules of 2017, In-Vitro Diagnostics (IVD) devices are categorized into four distinct classes as per the Medical Device Rules (MDR). These classes, governed by the Central Drugs Standard Control Organization (CDSCO), are classified as follows:
Class A IVD devices: These devices are deemed to pose low risk and are typically exempt from stringent regulatory control. Examples include laboratory reagents and solutions utilized for testing.
Class B IVD devices: Devices falling under this category are considered to carry a moderate level of risk and may require limited regulatory oversight. Examples encompass pregnancy test kits, blood glucose test strips, and urine analysis test strips.
Class C IVD devices: Devices categorized as Class C are regarded as posing a high risk and thus necessitate more rigorous regulation. Examples include HIV testing kits, hepatitis testing kits, and cancer diagnostic kits.
Class D IVD devices: Devices classified as Class D are deemed to carry the highest level of risk and therefore require the most extensive regulatory scrutiny. Examples encompass genetic testing kits, companion diagnostics, and specific laboratory-developed tests.